The Biochemistry of Breathing Fire
Could dragons do what no known organism has done before?

Could dragons do what no known organism has done before?
Breathing fire wouldn’t be easy. First, a dragon’s body would need to produce and store some volatile substance. Then, it would need to eject that substance at high speeds, kind of like burping but in a more prolonged and organized manner. Finally, the dragon would need to ignite that fuel as it left its body. The dragon would also need to be internally and externally fireproof to survive its own flames. Is such a setup even possible? Let’s find out.
Unlike other Snipette pieces, this one involves quite a bit of math and biochemical equations. If you want to skip these sections, feel free to use the "math-free" toggle below or watch out for icons on the sides of the paragraphs:
This is the second of a five-part series on the evolution and biology of (hypothetical) dragons. Each part is a self-contained unit, so you could either read them all or simply dive in where you like.
First, the fuel. There’s already a flammable compound that forms inside many animals: methane. Methane is produced by microbes in the intestines of many animals as they break down partially digested food. We’ve established that dragons would likely evolve air sacs to store the oxygen needed to power their muscles. Perhaps similar sacs could evolve to gather methane instead. We can determine how much methane our dragon would need to store by comparing its output to that of a flamethrower.
On a full tank of fuel, the X15 flamethrower can shoot fire up to 45 feet(14 m) for a full minute. Any flammable liquid works, but it gets its best range using a mixture of 75% gasoline and 25% kerosene. If we compute the energy released by burning a full 13.25-liter tank of this mixture, we get approximately 460 megajoules.
Let’s say our dragon can breathe fire for up to a minute with the same power output as the X15(or considerably longer with a fraction of the power output). That means it needs to release 460 megajoules of energy, which is the equivalent of burning 8.3 kilograms of methane.
And here comes our problem. At a body temperature of 100°F and normal atmospheric pressure, that much methane would take up 13,000 litres (13 cubic meters) of space. That’s 73% the volume of a typical grey whale, for the fuel sac alone. Definitely too much, even for a dragon!
The dragon could compress it down to 970 litres—about a fifth the size of an elephant—but that would require a lot of pressure: 200 psi. Even if the methane sac evolved to be roughly spherical to minimize its surface area, the dragon would still need to exert about 719 US tons of force to compress it. To put that in perspective, an elephant can carry about 9.9 US tons.
On a full tank of fuel, the X15 flamethrower can shoot fire up to 45 feet(14 m) for a full minute. Where the flamethrower uses efficient liquids like gasoline and kerosene, our dragon has to make do with methane. And therein lies our problem. To achieve one minute’s worth of an X15’s power output, our dragon would need space for an enormous amount of methane. I’m talking 13,000 litres! That’s 73% the volume of a typical grey whale for the fuel sac alone.
Perhaps the dragon could compress the methane down to a fifth the size of an elephant, but then it would need to exert at least 719 US tons of force. To put that in perspective, an elephant can carry about 9.9 US tons.
Well, not too bad for our first dragon. There are some obvious shortcomings, but hey, at least we made it breathe fire! (We’ll talk about ignition in a bit.) No dragon is complete without a name, so perhaps we can call our large, unwieldy creature the Megalomethane.

The Megalomethane would face problems other than its outlandish strength. Its large size would create challenging drag forces during flight, and its gaseous methane would spread out when expelled. These quick-to-dissipate fire attacks would be difficult to aim. All around, not a very practical anatomy. We need a more efficient method of creating flames. For instance, what if our dragon could convert its gaseous methane into liquid methanol.
Methanol would take up much less space than methane (only 25.8 litres), and as a liquid, it would be much easier to expel in a controlled manner. So, is it possible for a living being to convert methane to methanol inside itself? Short answer: it’s complicated.
The chemical equation for creating methanol from methane is pretty simple. Combine two moles of methane with one mole of oxygen gas, and you’ll get two moles of methanol about 5% of the time — which sounds dismal, but it means you’ll get there once every 20 tries.
Methanol production generates heat, which will have to leave our dragon’s body somehow. A fireproof dragon would have an incredibly well-insulated body, so the heat should be released primarily via convection. In other words, air from inside the dragon would be expelled carrying heat with it. Our dragon would need to expel as much of this heat each day as it produced. The amount of heat it could expel per day depends on the surface area of the methanol formation sac. Since this reaction requires at least 50 atmospheres of pressure, the surface area of the fuel sac will also tell us how much force our dragon would need to exert on it.
With the right formula, we can relate the dragon’s strength to the minimum amount of time it would take to produce enough methanol for its one-minute burst of flames without overheating.
The reaction would happen at 100°F (38℃), a reasonable body temperature for a living thing, and requires a pressure of at least 50 times that of Earth’s atmosphere—which sounds like a lot but is only about half the pressure that would be exerted by a 45-kg person wearing stiletto heels: not something you’d want to bear, but not unthinkable either. The reaction shouldn’t release more than 63 kilojoules of energy per mole of methanol produced under these conditions. Together, these factors relate the dragon’s strength to the minimum amount of time it would take to produce enough methanol for a minute-long burst of fire without overheating.
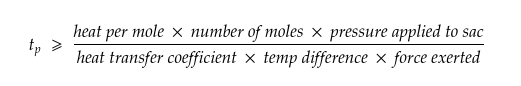
Which, if we plug in the values, becomes,
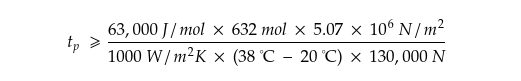
That makes,
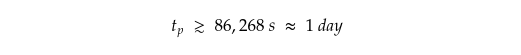
One day is how long our dragon would take to synthesise the required amount of methanol.
With the right formula, we can relate the dragon’s strength to the minimum amount of time it would take to produce enough methanol for its one-minute burst of flames without overheating.
After plugging in the numbers—the pressure applied to the sac, the temperature difference between the sac and the outside world, and so on—we can determine how strong our dragon would need to be to make enough methanol for a one-minute burst of X15-level firepower.
The answer? 86,268 seconds, or about one day. Not too bad for a dragon.
Flipping the formula around, let’s see how strong a dragon needs to be to produce enough methanol in a day. If our dragon made 436 megajoules worth of methanol a day, assuming optimum convective heat transfer, it would need to apply over 130,000 newtons of force to its gas sac. That still leaves our dragon 1.5 times as strong as an elephant, but at least its fire attacks would be more precise than the Megalomethane.
For this calculation, I assumed that 63,000 joules of heat would be released per mole of methanol produced. In actuality, the reaction would produce less heat than this, because that’s the amount it would release around room temperature (77 °F or 25 °C), not dragon body temperature (100°F or 38 °C). There would also be endothermic reactions happening in the fuel sac that would absorb some of the heat. That means our dragon could actually produce methanol even faster with the same 130,000-newton compressive force or at the same rate with less compressive force.
Things are even a bit better, because I calculated this assuming the methanol was produced at room temperature. In actuality, the dragon’s body temperature would be a bit higher, meaning it could either produce methanol even faster with the same elephant-and-a-half force, or produce the same amount of methanol while putting a bit less stress on its muscles.
So what would it mean for our dragon to produce this much methanol a day? To do so would require 632 moles of methane a day. The gassiest animals are cows, which at most produce about 0.3 kilograms of methane per day. Given the 5% success rate of converting methane to methanol, that means our dragon would need to produce methane 676 times as fast as a cow.
So what would it mean for our dragon to produce this much methanol a day? According to the math, it would need to produce methane 676 times faster than cows, which are already the gassiest animals real-world animals.
Of course, not all the methane would need to come from our dragon. In some scenarios, it could also inhale methane from the air. For example, it could live somewhere like a bog where the air has a high concentration of methane. However, in such a habitat, it would be in constant danger of setting its surroundings on fire. A better option would be to visit bogs regularly and fuel up.
Here’s another compelling idea: what if our dragon actually kept livestock around itself in a poorly ventilated space, say, a cave? If it captured plenty of cows or other methane-rich prey, kept them trapped in a cave, and let them pass gas until they died of starvation, it could inhale their methane to help fuel its firebreath. Then, it could eat the cows once they died. This diet, though gruesome, would also provide our dragon with another helpful substance: iron.
The last thing we need for our dragon to fuel up on methanol is a catalyst. Catalysts are substances that allow chemical reactions to occur at a faster rate or under different conditions than they normally would.
It just so happens that iron embedded in graphene is a catalyst for the reaction of methane and oxygen to form methanol. It even allows this reaction to occur at room temperature. Meat is rich in iron, so a dragon with a meaty diet might be able to use some of the heme iron it absorbed from meat to create its graphene catalyst.
The only other ingredient for this graphene would carbon, which our dragon could get from pretty much any organic substance. Graphene is a super-thin material; just one atom thick. It’s also the strongest material known to science, so using graphene to line the dragon’s gas sac would help it withstand the immense pressure being put on it by the beast’s muscles. Graphene is also great at conducting heat, so it wouldn’t prevent the gas sac from being cooled.
As a bonus, graphene provides an awesome name for our hypothetical dragon: the Graphene-Crested Skyflame.
Now our dragon has its fuel, but it’s not all that useful unless there’s a way to ignite it. Perhaps it could possess some kind of natural flint in its throat or mouth and scrape it together to spark a flame. Or, it could ingest small rocks, like some birds do, and scrape those together for ignition.
Or, the dragon could use the same method of ignition as the X15 flamethrower. It uses compressed carbon dioxide to eject fuel so fast it ignites due to friction with its container. It uses carbon dioxide because it’s non-flammable. You don’t want an accidental flare-up in the wrong place! The X15 specifically uses a 20-ounce tank of carbon dioxide pressurized at 800 pounds per square inch. Our dragon could have another gas sac with a volume of 6 litres for carbon dioxide and use its superstrong muscles to compress the gas. However, its strength would need to increase to 9.8 times that of an elephant to get the same 45-foot range as the X15.
Whether it uses methane or methanol, our firebreathing dragon will need to be disproportionately strong for its size. This incredible strength could arise if our dragon produced more strength-enhancing hormones and fewer strength inhibitors than other animals. Additionally, it could have muscles similar to arthropods and molluscs. These organisms’ muscles can produce more force per unit area than vertebrate muscle. Exceptionally efficient muscles could also help our dragon fly with the added weight of its fuel.
So are we done? Firebreathing is possible!
Wait, not so fast.
There are a few biochemistry issues that need to be addressed. For starters, methanol is toxic, and it can be absorbed through contact with an animal’s body.
When this happens, methanol is oxidised by the enzyme alcohol dehydrogenase to form formaldehyde. Afterwards, some of the formaldehyde is converted to formic acid by another enzyme, aldehyde dehydrogenase. This formic acid can build up in the blood, inhibit mitochondria, and cause problems like nerve damage. What’s worse, this isn’t the only toxin our methanol-spewing dragon would be producing. Remember those secondary endothermic reactions I mentioned, which capture some of the heat generated in the process of creating methanol? Well, those reactions also create carbon monoxide, of ‘carbon monoxide poisoning’ fame.
When this happens, the methanol is oxidised by a certain enzyme to form formaldehyde, which is then converted again to formic acid. This formic acid can build up in the blood, stop parts of the cells from functioning, and cause problems like nerve damage. What’s worse, this isn’t the only toxin out methanol-spewing dragon would be producing. Remember those secondary reactions I mentioned, that capture some of the dragon’s heat? Well, those reactions also create carbon monoxide, of ‘carbon monoxide poisoning’ fame.
Yikes! Does this mean our dragon’s greatest weapon would also be its downfall? Not necessarily.
Fortunately, there’s a chemical called fomepizole that inhibits alcohol dehydrogenase and prevents cells from metabolizing methanol. This chemical is composed of carbon, nitrogen, and hydrogen, which are all common in organic chemistry. Perhaps our dragon could produce this chemical naturally to prevent methanol poisoning.
Fortunately, there’s a chemical called fomezipole that stops the enzyme in question from forming formaldehyde. Fomepizole is made up of commonly available carbon, nitrogen and hydrogen, all of which are abundant in the natural world. So perhaps our dragon could produce this chemical naturally, to protect itself from methanol poisoning.
As for carbon monoxide, there are actually microbes that can consume this toxic gas for energy and produce organic compounds in their place. Perhaps our dragon’s microbiome could include such microbes to protect it from carbon monoxide poisoning. In fact, some of these microbes use a process called ‘methanogenesis’, which takes carbon monoxide and water and converts them into methane and carbon dioxide.
Methane and carbon dioxide are both products that our dragon could use to fuel and propel its fire attacks.
There is one final snare here, which is that methanogenesis generates heat and would create more heat for our dragon to expel via convection. That means a larger fuel sac and a bit more muscle exertion. Luckily, not all the carbon monoxide would need to be converted. The microbes would only have to convert that which would otherwise be absorbed by the cells lining the dragon’s interior. This factor keeps the ‘extra heat to manage’ levels from increasing detrimentally.
And that is how, with the right microbiome, our dragon could literally turn poison into power.
What’s next? There’s more than one way to spark a flame, and more than one way a living organism could spew flames. Watch out for the next instalment of this five-part series, where we look at an alternate fuel source that another hypothetical dragon species could utilize.